Metal-Ligand Chemistry
Che’s seminal research in Reactive Metal-Ligand Multiple Bonded Complexes and d8/d10 metal complexes has led to novel excited state atom transfer catalysis, selective hydrocarbon functionalization reactions, practical phosphorescent and metal-assisted TADF emitters, unique functional supramolecular metal assemblies, and anti-cancer metal medicines for combating metastatic cancers.
Iron Catalysis
In collaboration with Shanghai Institute of Organic Chemistry (SIOC), we have been working on the development of practical iron-catalyzed organic transformation reactions. Development of iron catalysts, compared with other transition metal catalysts, is of most significance because of (1) the high earth abundance of iron, which limits preparation cost of the iron catalysts to an affordable level; (2) the excellent biocompatibility of iron complexes, which renders iron catalysts the best for the synthesis of bioactive molecules; (3) the robust catalytic activity of iron complexes towards organic transformations with excellent selectivity. We envision that the development of iron catalysis is the increasing trend in the academia, and this would also be the future trend to the chemical industry as a plausible solution to the pollution problem faced in the current industrial practices. The major challenge of this study would be the synthesis and characterization of the high-valent iron-oxo and/or iron-nitrido species, which are highly reactive for selective bond-forming reactions.
Atom/Group Transfer Reactions and C–H Functionalization
Direct and selective C–H bond functionalization is appealing for organic synthesis because of the ubiquity of C–H bonds in organic molecules, and this strategy provides promising potential in streamlining the synthetic routes of useful chemicals, including molecules with biological or pharmaceutical activities, new materials and reaction intermediates towards these purposes. This strategy also coincides with the principles of Green Chemistry by elevating atom and energy economy with minimum production of wastes, which bring the development of academic research and synthetic chemical industry towards a sustainable future. With our continuing effort on metal-ligand multiple bonds, we focus on the carbene, nitrene and oxygen transfer/insertion reactions. Our catalytic systems allow selective carbene and nitrene transfer reactions with desired product yields up to 99%, which advances the development of atom and group transfer reactions.
Application on Synthesis of Natural Products
We have also applied these atom and group transfer reactions to the formal synthesis of natural products and bioactive molecules, showing the potential of these reactions in practical organic syntheses.
01 /Reactive Metal-ligand Multiple-bonded Complexes
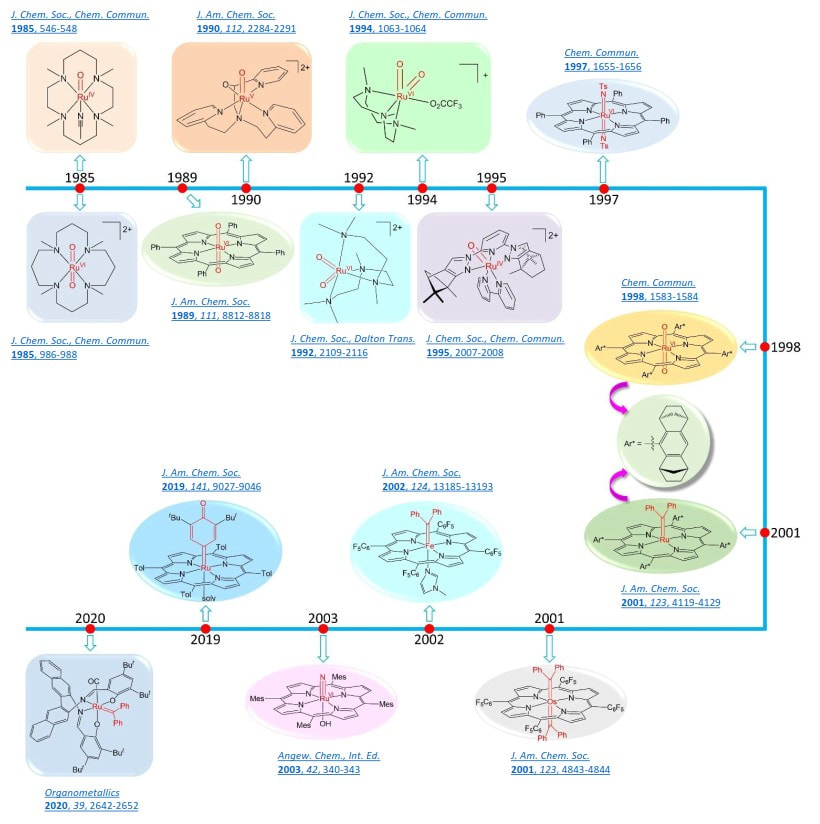
With our continuing effort to study metal-ligand multiple bonds and their applications in transition metal catalysis for practical organic transformations, various metal catalysts have been developed for oxygen atom and carbene or nitrene group transfer/insertion reactions. Through judicious choice of ligands and metal ions, we are able to synthesize and isolate diverse classes of metal-oxygen, -nitrogen and -carbon multiply bonded complexes including highly reactive ones for understanding the mechanisms of metal-catalyzed atom and group transfer to organic substrates at the molecular level via spectroscopic measurements and/or by DFT calculations. Useful structural information has been obtained for the rational design of more robust metal catalysts with higher selectivity.
02 /Phosphorescent d8/d10 materials
Che pioneered the field of phosphorescent d8/d10 metal emitters, including the molecular design studies, excited state dynamics and fundamental non-covalent metal-metal and metal-ligand interactions in directing supramolecular self-assembly. His work on [Pt2(pop)4]4- (with Gray) and his [Au2(dppm)2]2+ paper (published in 1990) opened up the field of gold/platinum photo-catalysis involving C-H and C-X bond activation via excited state inner-sphere atom transfer reactions. [Au2(dppm)2]2+ photochemistry has been extensively studied by different organic chemists around the world for the practical synthesis of polycyclic compounds under visible light irradiation. [Pt2(pop)4]4- photochemistry has been used for practical acceptor-free photochemical dehydrogenation reactions. He developed novel robust d8 and d10 phosphorescent metal complexes as well as metal-TADF emitters with large radiative decay and low non-radiative decay rates. He and Ma first reported the application of phosphorescent metal complexes in OLEDs. He led the development of robust platinum(II) emitters with large radiative decay rates, such as the tetradentate [Pt(ONCN)] system, which was licensed and subsequently developed in Samsung products. He first reported OLEDs with Cu emitters and recently Cu-TADF OLEDs with high EQE values and practical operating lifetimes. His molecular design research led to the development of strongly blue-green phosphorescent palladium(II) and gold(III) complexes with high emission quantum yields and long excited state lifetimes in solutions required for photo-functional applications under ambient conditions. Che made seminal contributions to the origin and applications of closed-shell metal-metal interactions in ground state (repulsive due to Pauli repulsion) and excited state (attractive due to metal-metal bonding) d8 and d10 complexes. He provides spectroscopic evidence for metal-metal bond (nds* -> (n+1)ps) excited states of d10 metal complexes. He early reported the 3MMLCT emission of dinuclear Pt(II) complexes in solution (in 1993) and used this emission as a reporter signal in molecular sensing (in 1998), subsequently leading to functional phosphorescent platinum(II) based supramolecular materials being extensively studied and developed by many scientists around the world. He pioneered the use of non-covalent intermolecular interactions of d8 metal complexes to create different nanoscale optoelectronic materials and synthesized diverse supramolecular copolymers and photonic waveguide hetero-structures through living supramolecular polymerization. These works opened up the field of functional molecular materials based on supramolecular assemblies of luminescent platinum complexes.
Platinium(II) emitters and metal-TADF emitters for OLED applications
Phosphorescent metal complexes have found profound applications in Material Science and that OLED materials for display and lighting are among their most prominent real-world applications because they enjoy a privileged electric-to-light conversion efficiency of up to unity over conventional fluorescent dyes. Our group has a long tradition and research excellence in design and development of original and proprietary phosphorescent platinum(II) complexes for OLED applications of practical interests and has established an array of robust and highly luminescent tridentate and tetradentate platinum(II) systems that emanate visible-light emissions from blue to green and red colors essential for full-color display as well as white and near infrared light emissions. A large majority of our platinum material patents have been licensed to the leading players in the industry, including Samsung and Merck.
The next generation of OLED emitters necessitates a full utilization of electrogenerated excitons with short radiative lifetimes to achieve both high light emission efficiency and operational stability, which are currently deficient especially for blue OLEDs. We envisage that metal emitters capable of harvesting singlet emission via thermally activated delay fluorescence (TADF) mechanism is a promising option. Our group in recent years has been in active exploration of novel and efficient metal-TADF emitters and has developed several unprecedented classes of strongly luminescent gold-, which has a much higher abundance than iridium and other noble metals, as well as earth-abundent tungsten-TADF emitters showing radiative lifetimes as short as sub-microsecond and PLQY >80%. The device efficiencies and stability of our gold(III)-TADF emitters are highly competitive with respect to the current best Ir(III) and Pt(II) emitters.
Photoredox catalysis with transition metal complexes
Photoredox catalysis has emerged as an efficient strategy for achieving solar-to-chemical energy conversion and selective functionalization of organic compounds with complexity under mild reaction conditions. In this regard, we have been developing various types of phosphorescent transition metal complexes including those of Pt(II), Au(III) and Pd(II) with long-lived excited states for catalyzing light-induced organic transformations such as oxidative C–H functionalization and reductive dehalogenation reactions. One of our directions is to employ coordinative-unsaturated metal complexes that activate C–X bonds (X = hydrogen, halogen) upon photo-excitation via an inner-sphere atom abstraction mechanism without the need for sacrificial electron donors/acceptors. Examples of these include binuclear Pt(II) diphosphite complexes (also known as “platinum pop”) and binuclear Au(I) diphosphine complexes.
Another direction is to develop photocatalysts based on earth-abundant metals such as copper, tungsten and iron. We reported the use of zwitterionic Cu(I) complexes for light-induced cross-dehydrogenative coupling reactions affording aza-Henry- and Mannich-type products. Air-stable luminescent W(VI) dioxo complexes supported by Schiff base or 8-hydroxyquinolinate ligands also demonstrated photocatalytic activity in oxidative cyanation of tertiary amines and hydroxylation of aryl boronic acids. Very recently, we reported iron porphyrin catalyzed light-driven C–H bond amination and alkene aziridination with organic azides.
03 /Anti-cancer d8/d10 Metal Complexes
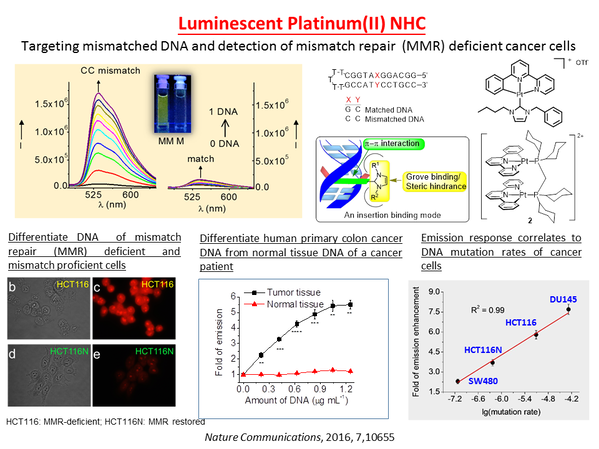
Che pioneered the development of d8/d10 metal complexes in cancer diagnostics and treatment. In his PNAS articles, he elegantly demonstrates how coordination chemistry, proteomics, multi-omics, bioinformatics, and molecular biology can be used to identify important molecular targets for anti-cancer metal complexes. He discovered that gold(III) porphyrin and d8 (pincer)metal complexes are unique sources of molecular scaffolds and drug leads that exhibit potent anti-angiogenic, anti-invasive and anti-migration properties against metastatic cancer cells. He demonstrated that these coordination complexes can provide an untapped source of chemical space that fits tightly into the binding pocket of important cancer-promoting proteins such as Hsp60, which is highly expressed in metastatic cancer cells and currently lacks effective inhibitors. He pioneered the development of phosphorescent platinum(II) and gold(III) complexes for the recognition and detection of nucleic acids, mismatched DNAs and RNAs.
Innovative Gold/Platinum Anticancer Medicines and Theranostics
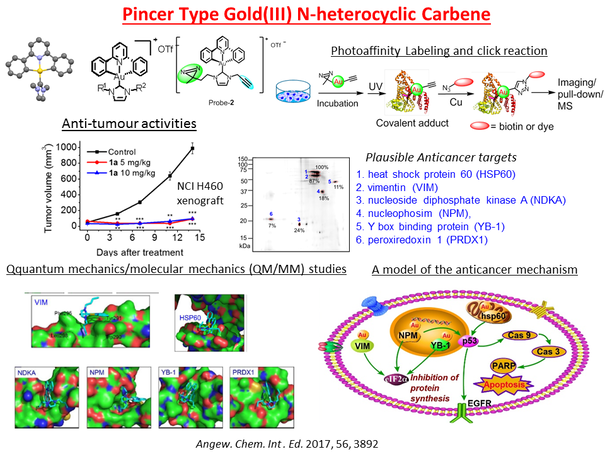
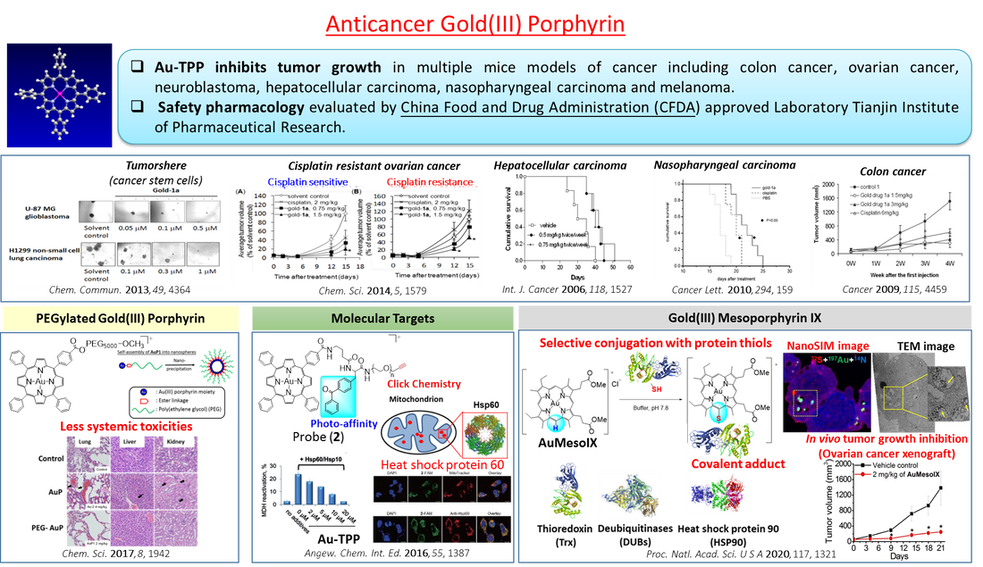
The clinical success of cis-platin has led to tremendous advances in metal coordination compounds for anti-cancer applications. In particular, planar coordination geometry and coordination unsaturation of d8 and d10 metal ions render their metal complexes to be unique scaffolds for the design of new diagnosis and therapy for treatment of cancers. We have employed ligands with N donor atoms such as porphyrins and C donor atom(s) such as N-heterocyclic carbenes (NHC) to construct cationic gold(III), Au(I), Pd(II), Pt(II) and Ir(III) complexes with good stability and cell permeability under physiological conditions. NHC ligands are able to stabilize metal ions against demetallation and render the metal complexes to be strongly emissive in solutions through suppression of excited state structural distortion. The rich luminescent properties of metal-N-heterocyclic carbene complexes are convenient spectroscopic handles for tracking of the complexes inside the cells and can also be used for the detection of biomolecular structures of relevance to cancers such as mismatched DNA. Some of these complexes exhibit potent cytotoxicity toward cancer cells and in vivo anti-tumor activities in multiple mice models of cancer including the cis-platin resistant and the metastatic ones. The in vivo metabolism of some of the anti-cancer metal complexes has been elucidated. Chemical formulation strategies of bioconjugation and encapsulation were used to deliver the cytotoxic anti-cancer metal complexes to the tumors with an objective to lower the toxic side effects. We have also applied click chemistry and photo-affinity probes, cellular thermal-shift proteomics and transcriptomic profiling to demonstrate that the anti-cancer metal complexes engage a number of molecular targets related to cancer progression and metastasis. Recently, we uncovered that for some gold(III) porphyrin scaffolds, the periphery of the porphyrin ligands can be activated by electrophilic gold center to selectively form covalent adducts with protein thiols. This is a new mode of biomolecular interaction that can be exploited for anti-cancer applications.
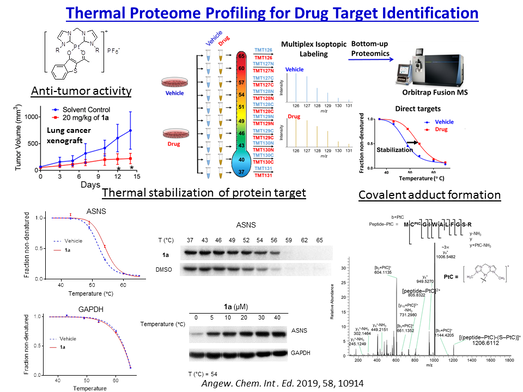
Proteomics and Drug Targets Identification
The identification of molecular targets of the bioactive compounds is crucial for realizing therapeutic applications. Our drug discovery program has established technology platforms employing transcriptiomics (next generation sequencing), proteomics (high throughput biological mass spectrometry), and bioinformatics (connectivity mapping, key-node analysis) in the elucidation of mechanisms of action of anticancer-active, metal compounds and natural products. Furthermore, we develop chemical probes (photoaffinity labeling, click chemistry, fluorescent compounds) for various bioactive compounds to identify the direct molecular targets. The target engagement of anti-cancer drugs in intact cells can also be investigated using probe-free methods such as those based on ligand-induced thermal stabilization of target proteins which, upon binding with the ligands, become more stable against heat-induced unfolding or precipitation even under cellular conditions. In combination with MS-based multiplexed quantitative proteomics analysis of the soluble proteins after thermal denaturation, the proteins engaged by the compounds of interest can be profiled in terms of the shift in the melting temperatures. Using these approaches, we have identified the molecular targets of a number of anti-cancer metal compounds and natural products with the drug-target interactions and downstream cellular mechanisms of action elucidated.
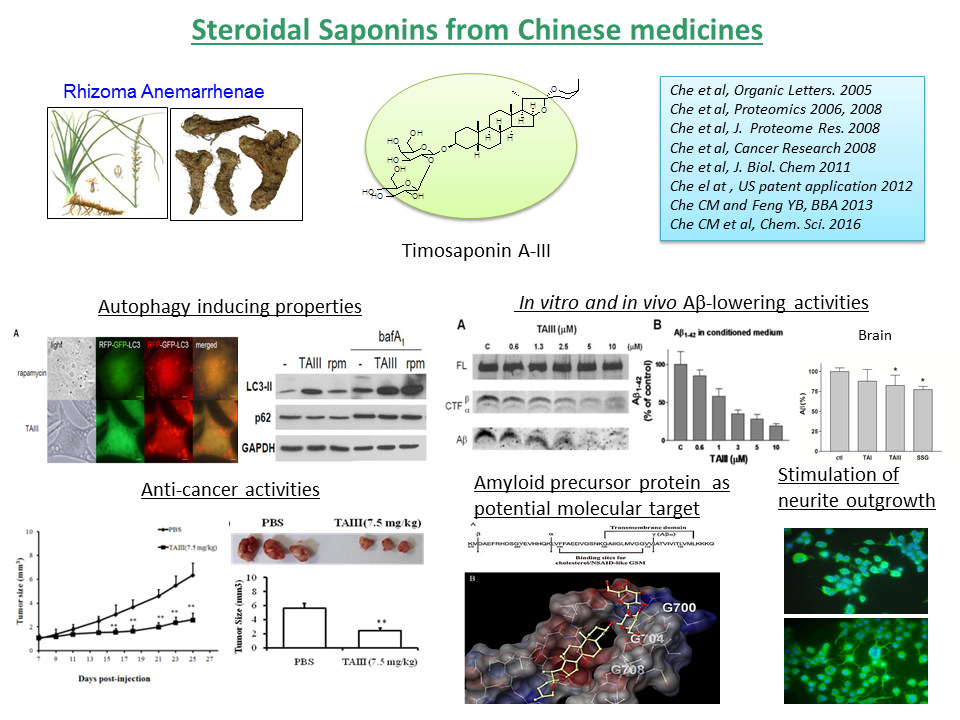
Natural Products from Chinese Medicines
Natural products, including those from Traditional Chinese medicines (TCM), are a significant source of lead compounds for drug discovery. Built on the foundation of R&D projects of drug discovery, we have identified a number of bioactive lead compounds from Chinese medicines with potential for treatment of cancer, neurodegenerative diseases and osteoporosis. Many of these lead compounds are also representative ingredients in the herbs found in prescribed formulae of Chinese medicine, whereas some were newly found to exhibit anticancer properties in pilot studies. Currently, we are developing several novel compounds showing in vivo inhibitory activities against mice cancer xenograft, and they will be further chemically modified and formulated to enhance the anticancer properties. Besides, purified compounds from Chinese medicines, Chinese Medicinal Formulations of mixed herbs (Fu Fang) are also investigated with an interest to apply phytochemical analysis and system biology for understanding the therapeutic properties. We are interested in co-develop Fu Fang which have been prescribed to cancer patients with good cure rate and safety in collaboration with the stakeholder company of the formulation. A multi-omics analysis is underway to decipher complex nature of therapeutic actions of the Fu Fang as well as to provide chemical fingerprint and biological response signature for quality analysis.